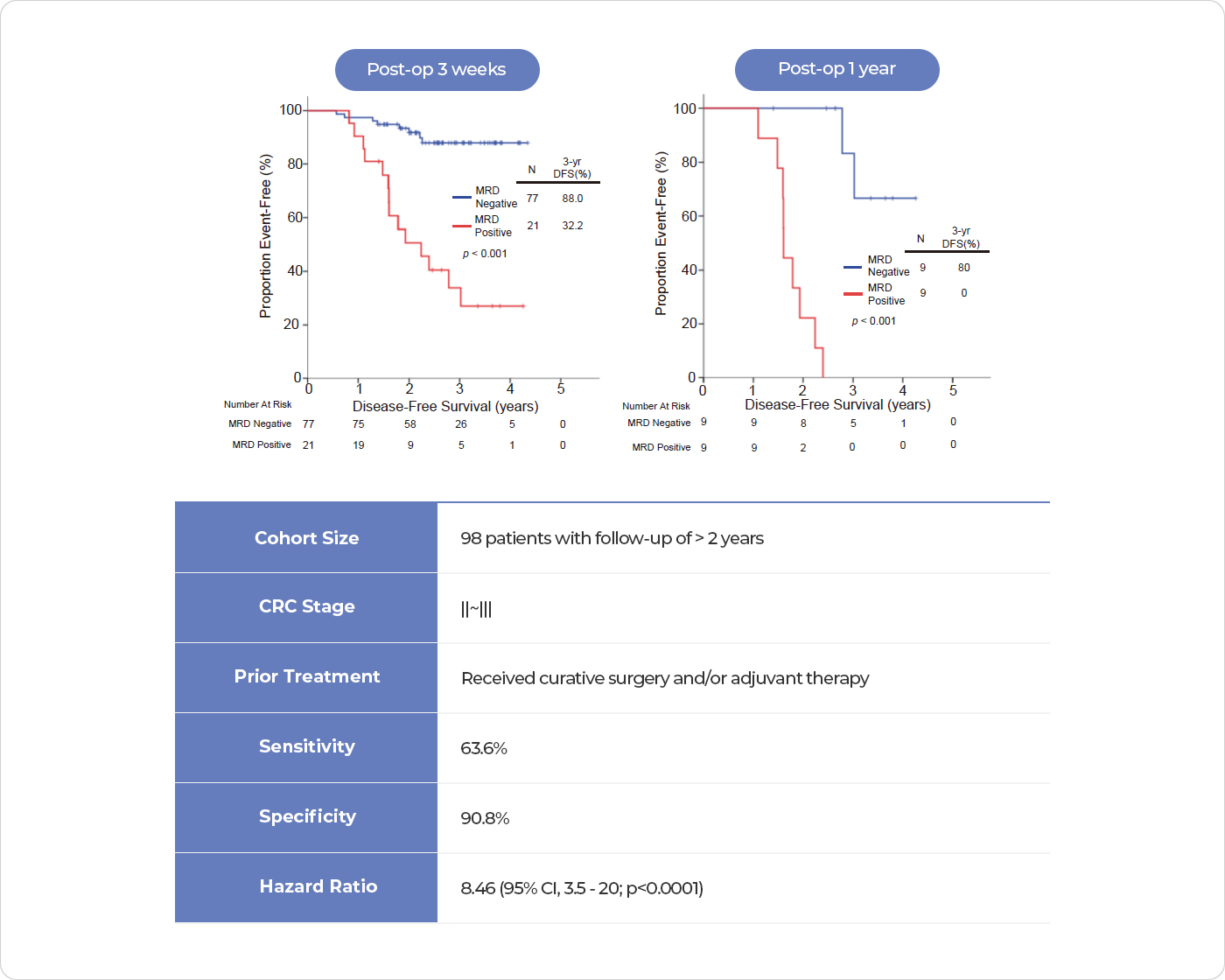
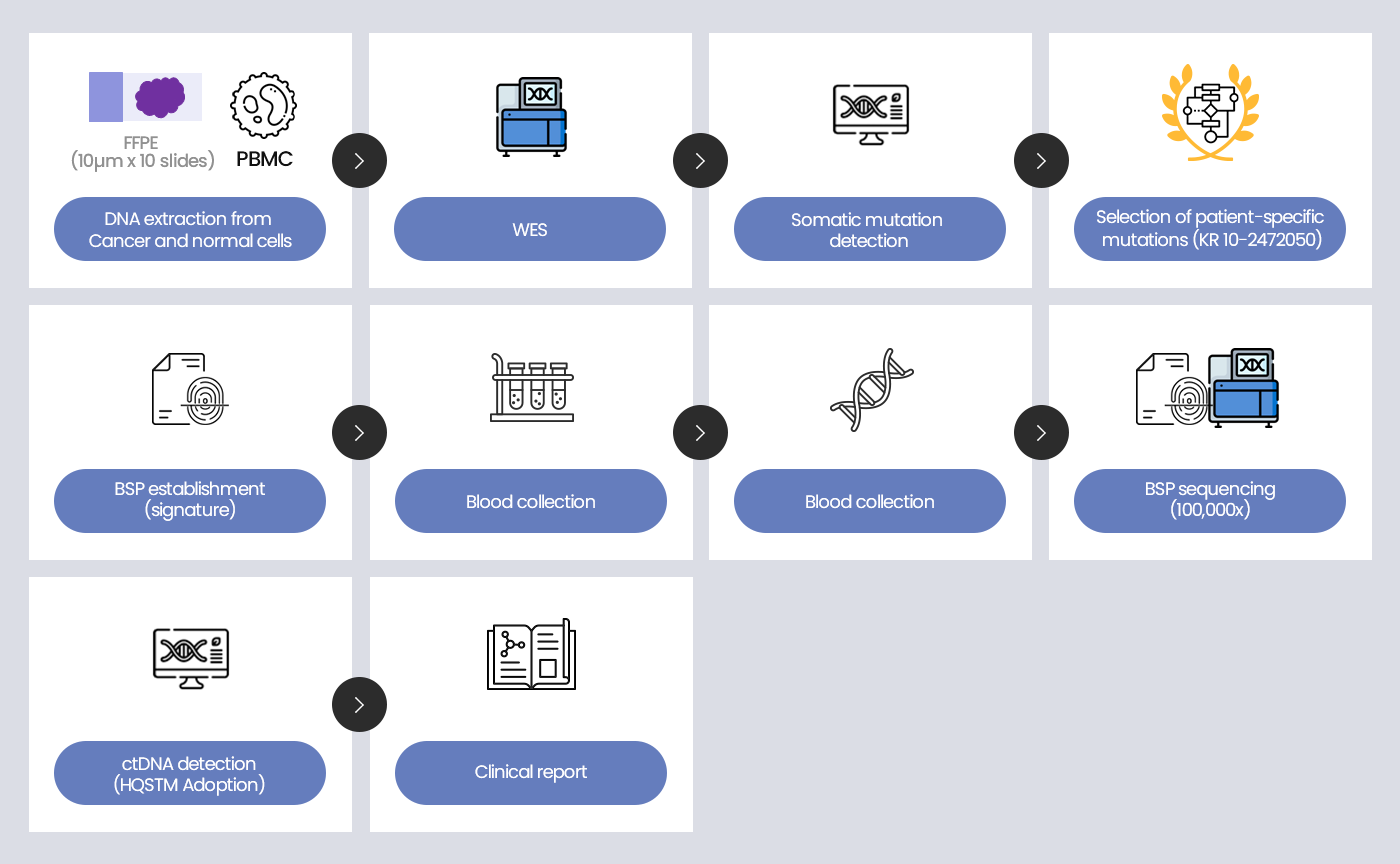
CancerDetect™ is our proprietary liquid biopsy platform tailored for the early detection of cancer recurrence post-surgery. This innovative technology pinpoints unique genetic mutations in surgically removed cancer tissues, laying the groundwork for a personalized genetic panel. The custom panel is designed for the meticulous monitoring of Minimal Residual Disease (MRD), postoperatively.
By employing this personalized genetic panel testing, we can forecast potential cancer relapse months in advance compared to traditional imaging techniques. Our forward-thinking strategy ensures the early identification of recurrences, allowing for prompt adjustments in treatment strategies based on test results. Ultimately, CancerDetect™ is pivotal in improving patient outcomes by anticipating cancer progression and fine-tuning chemotherapy regimens.
| Assay Type | Tumor tissue sequencing Targeted hybrid capture NGS based on proprietary HQS™ technology |
|---|---|
| Sample Requirement | Tissue : FFPE (10μm x 10 sections) or FF Blood : >20mL of whole blood |
| Targets | Customized panel of up to 100 targets |
| Detected Alterations | SNVs and Indels |
| Limit of Detection | 0.005% ~ 0.0005% ctDNA fraction |
-
Cancer-related variant detection
-

Tissue-informed personalized panel design
-

Recurrence monitoring